Learning Outcomes
By the end of this lesson, students should be able to:
i. Explain the nucleophilic addition reactions of aldehydes and ketones.
ii. Identify the factors affecting the reactivity of aldehydes and ketones towards nucleophilic addition reactions.
iii. Describe the different types of nucleophilic addition reactions undergone by aldehydes and ketones, including hydration, addition of alcohols, and addition of hydrogen cyanide.
iv. Compare the reactivity of aldehydes and ketones towards various nucleophiles.
Introduction
Aldehydes and ketones are carbonyl compounds, meaning they contain a carbon atom double-bonded to an oxygen atom. This carbonyl group is the reactive center in these molecules, making them susceptible to nucleophilic addition reactions.
i. Nucleophilic Addition Reactions of Aldehydes and Ketones
Nucleophilic addition reactions involve the addition of a nucleophile (a species with an electron-rich atom) to the electrophilic carbonyl carbon of an aldehyde or ketone. The general mechanism for nucleophilic addition to carbonyl compounds is shown below:
Nucleophile + RCOR' → Adduct
In this reaction, the nucleophile attacks the electrophilic carbonyl carbon, forming a tetrahedral adduct. The tetrahedral adduct can then undergo further reactions, leading to the formation of various products.
ii. Factors Affecting the Reactivity of Aldehydes and Ketones
Several factors influence the reactivity of aldehydes and ketones towards nucleophilic addition reactions. These factors include:
Steric Hindrance: Bulkier substituents around the carbonyl group hinder the approach of the nucleophile, decreasing reactivity.
Electronic Effects: Electron-donating groups (EDGs) increase the electron density on the carbonyl carbon, making it less electrophilic and reducing reactivity. Conversely, electron-withdrawing groups (EWGs) decrease electron density, making the carbonyl carbon more electrophilic and increasing reactivity.
Nucleophilicity of the Nucleophile: Stronger nucleophiles react more readily with aldehydes and ketones.
iii. Types of Nucleophilic Addition Reactions
Aldehydes and ketones undergo various nucleophilic addition reactions, including:
Hydration: Aldehydes and ketones react with water in the presence of an acid catalyst to form geminal diols.
Addition of Alcohols: Aldehydes and ketones react with alcohols to form hemiacetals or acetals, depending on the reaction conditions.
Addition of Hydrogen Cyanide: Aldehydes and ketones react with hydrogen cyanide (HCN) to form cyanohydrins.
iv. Comparison of Reactivity
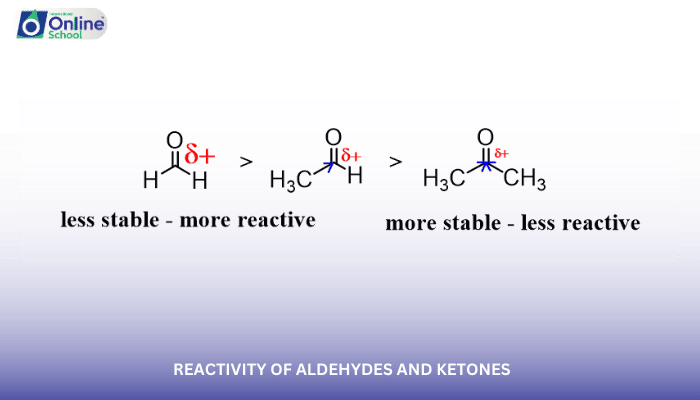
Aldehydes are generally more reactive towards nucleophilic addition reactions than ketones. This difference in reactivity is due to the presence of a hydrogen atom on the carbonyl carbon of aldehydes, which can stabilize the intermediate tetrahedral adduct. Ketones, lacking this hydrogen atom, have less stable intermediates and are therefore less reactive.
The reactivity of aldehydes and ketones is primarily determined by the nucleophilic addition reactions they undergo. These reactions are influenced by various factors, including steric hindrance, electronic effects, and nucleophilicity. Understanding the reactivity of aldehydes and ketones is essential for predicting their behavior in various chemical reactions and for their synthetic applications.